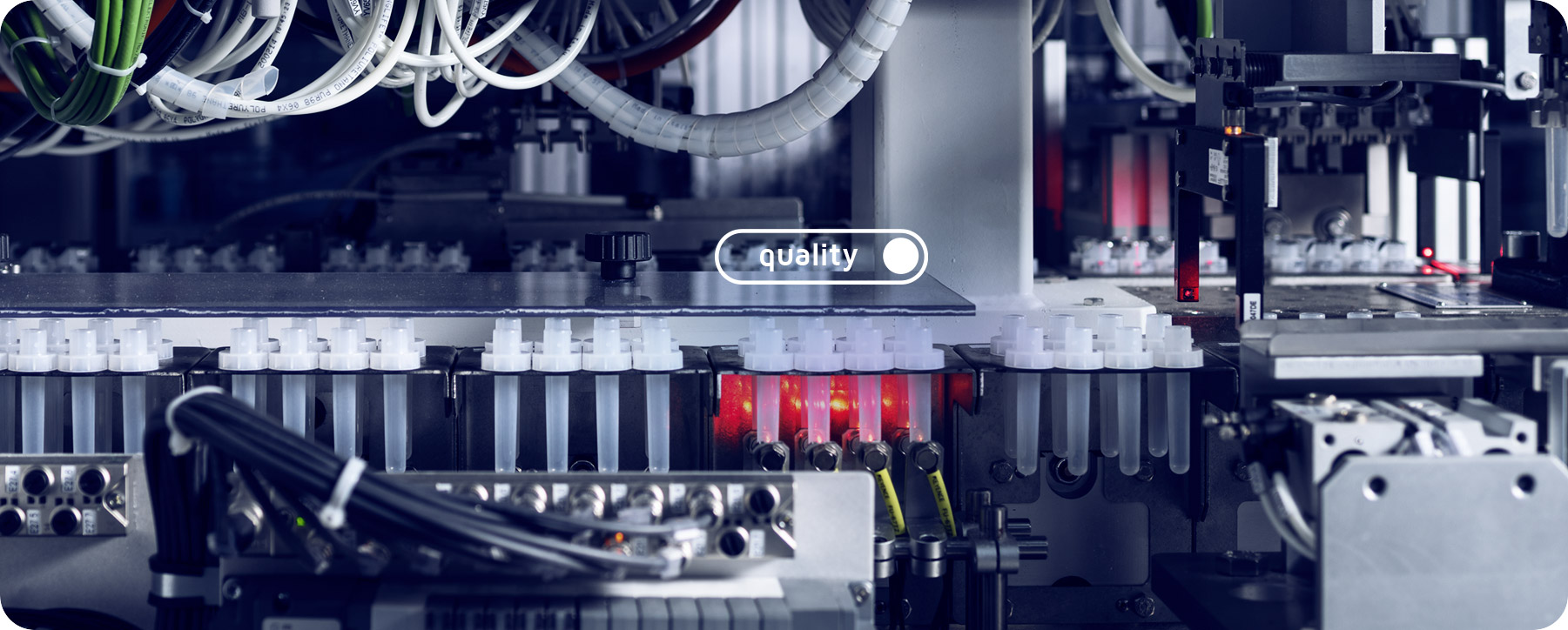
Guaranteed quality.
Always.
In Vitro Diagnostic Medical Devices (IVD) are an essential part of healthcare. For this reason, Anicrin constantly and accurately controls the production of incoming and outgoing materials.
The quality of the products is guaranteed by a sophisticated production process, totally internal and in a continuous cycle over 24 hours. Thanks to an air filtering and conditioning system, constant temperature and humidity levels are maintained within the ANICRIN production department. The average microbiotic load is monitored through tests performed by independent qualified laboratories.
The efficiency of the production system and attention to the quality of the materials have allowed ANICRIN to obtain certifications according to the standards:
UNI EN ISO 9001: 2005 - Quality management systems
UNI CEI EN ISO 13485: 2016 - Medical devices, Quality management systems


 UNI EN ISO 13485:2016
UNI EN ISO 13485:2016
 UNI EN ISO 9001:2015
UNI EN ISO 9001:2015